Self-Administration Antigen Kit – 446 Units
€1.784,00
Product available only for professionals, health services, residences, health managers of sports facilities and companies authorised by their occupational risk prevention service.
| Self administration Units box | ||
|---|---|---|
| Number of units | €/box | €/u. |
| 1 carton (446 u.) | 1,784 | 4 |
| 1 pallet (12 cartons – 5,352 u.) | 20,070 | 3.75 |
| 2 pallets (24 cartons – 10,704 u.) | 37,464 | 3.5 |
| 4 or more pallets (21,408+ u.) | Contact us | |
What’s inside?
Single dose box
The box contains a guide of use, the test cassette, a disposable sterile swab to collect the nasal sample, an extraction buffer tube to put the swab once the sample is collected and a biohazard waste bag.
You can use the carton box pre-cut hole to hold the extraction buffer tube on it while you do the procedure.

The single box product comes in cartons of 446 units.
When should the antigen test be used?
The SARS-CoV-2 Antigen Test Kit can detect an infection by SARS-CoV-2 in both symptomatic and asymptomatic patients.
Its use it’s recommended before travelling, an office visit, assisting an event, medical intervention… Particularly to distinguish the diagnosis of seasonal flu or a common cold from Covid-19.
How is it used?
Watch this video for information on how to do the procedure.
Reading the results
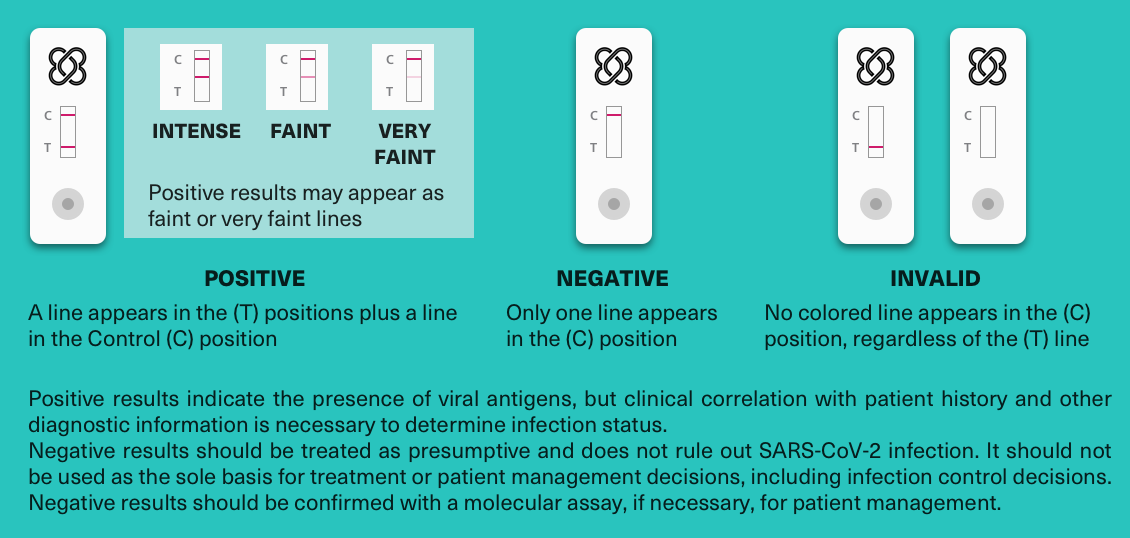
Read the results after 15 minutes, and before 20 minutes have passed. Positive results are indicated by a colored line next to the “T”. The control line “C” must always be visible for the test to be considered valid.
Actions
Positive results indicate the presence of viral antigens, but a clinical correlation with the patient’s history and other diagnostic information are necessary to determine the status of the infection.
A negative result should be treated as presumptive and does not rule out SARS-CoV-2 infection. It should not be used as the sole basis for determining treatment or clinical decisions. Negative results should be confirmed with a molecular assay if necessary for patient management.
The antigen test indicates if the user of the test has SARS-CoV-2 virus present in the mucous membranes of the anterior nasal area at a given moment, but it does not allow us to know the status or progression of the disease.
It is important to bear in mind that a patient with a positive result is contagious and therefore preventive isolation of both the subject and their circle of contacts is recommended.
Reliability

The Linkcare Antigen Kit has been clinically validated with very positive results in terms of sensitivity and specificity, as well as a negative predictive value of 100% and a positive predictive value of 98%.
Certificates
The self-administration tests have all the necessary certificates for their commercialisation in the European Union and it’s included in the EU common list of COVID-19 rapid antigen tests. Their sensitivity and specificity have been validated both in Europe and China.




